📊 By that publication date (July 24, 2025), we had already completed:
Treatments for 25 human cancer patients in China and at the Rheinfelden Clinic (Germany); 3 veterinary tumor cases.
Several clinical case reports, published here: 👉 View Case Reports on Intra-Tumoral ClO₂ Therapy
✅ These cases, completed before July 24, 2025, demonstrate that intratumoral chlorine dioxide therapy had real-world clinical data, directly contradicting the claim made in WIRED.

System Architecture

Responsible for the preparation and containment of the chlorine dioxide ablation agent.
This module ensures controlled concentration, stability, and safe handling prior to intratumoral delivery.
01-Ablation Agent Module
Enables precise intratumoral delivery of the ablation agent into tumor tissue.
The module supports controlled volume, rate, and localization during the ablation procedure.
02-Injection Control Module
Provides image-based planning and procedural guidance for intratumoral ablation.
This module assists clinicians in defining injection targets and optimizing procedural accuracy using imaging data.
03-Algorithmic Module
Self-Limited Reaction + Dual Local Mechanisms → Repeatable, System-Preserving Tumor Clearance
Why Intratumoral Chlorine Dioxide Enables Iterative Tumor Clearance
Diffusion–Reaction Self-Limiting Radius
Chlorine dioxide operates within a diffusion–reaction–coupled system.
Its effective activity naturally terminates within a stable radius of approximately 1–2 cm, with concentration decaying exponentially over distance.

Defines a hard spatial boundary for safety and control
Dual-Mechanism Local Ablation
Within the self-limited radius, chlorine dioxide produces a direct cytotoxic core while simultaneously disrupting abnormal tumor vasculature.
These two mechanisms reinforce each other without extending damage into surrounding normal tissue.
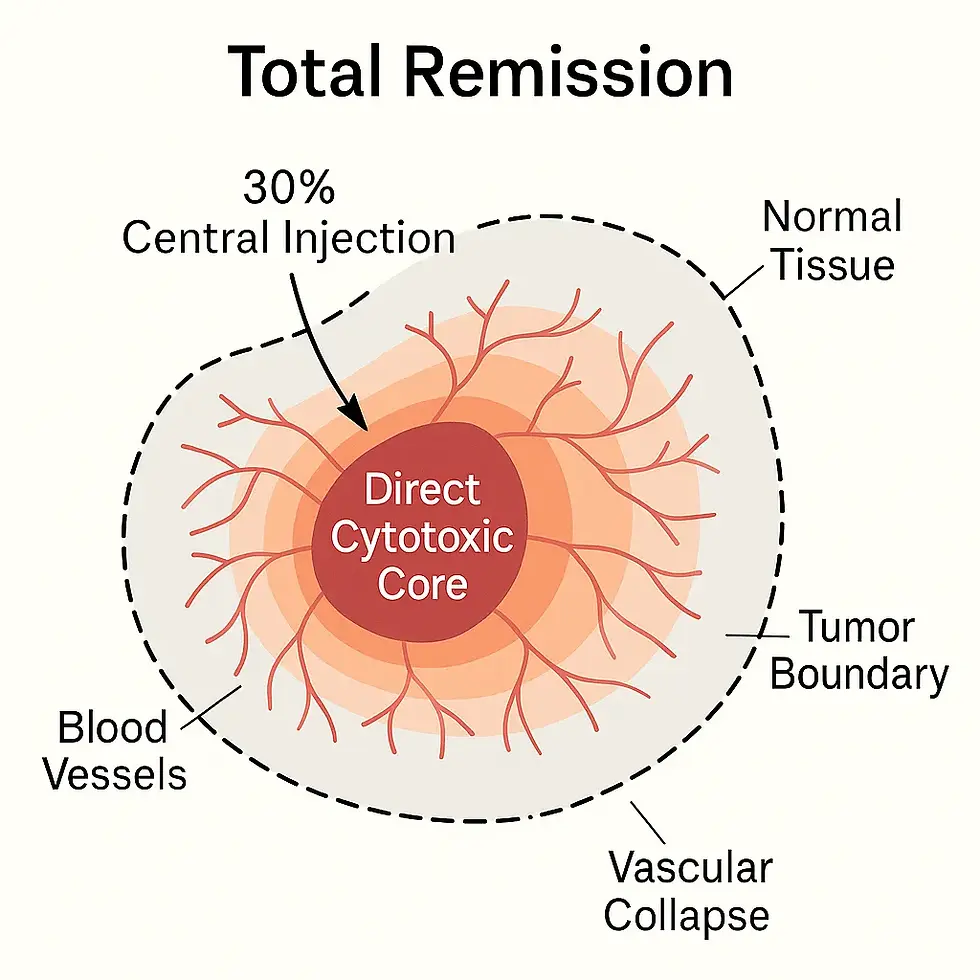
High local efficacy without systemic exposure or sealing tissue fate
Iterative Clearance Through Repeated Intervention
Because tissue is not driven into a terminal fibrotic state, treated regions remain accessible.
Repeated image-guided intratumoral injections allow progressive reduction of tumor burden, converging toward complete clearance over time.

Repeatable intervention enables convergence rather than one-shot sacrifice
Intratumoral Chlorine Dioxide Ablation System for Solid Tumors
This medical device system delivers chlorine dioxide directly into tumor tissue for localized ablation.
By focusing on intratumoral intervention, the system is intended to address solid tumors across different disease stages within structured medical protocols.

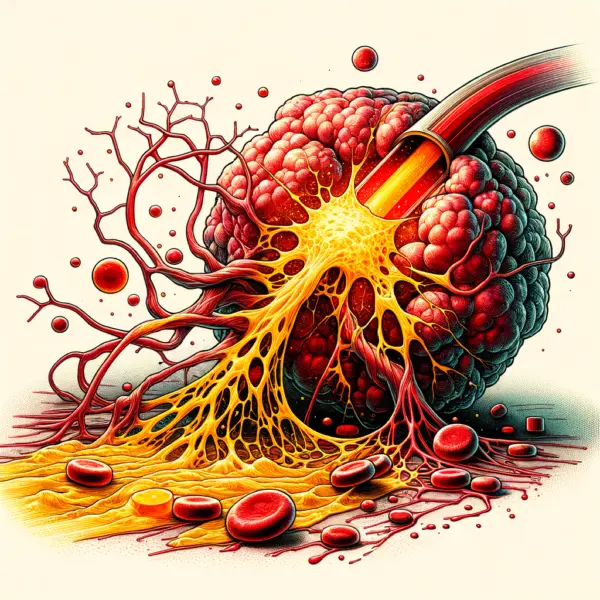
1. Direct Oxidative Destruction of Cancer Cells
2. Tumor Vascular Disruption
3. Reduction of Tumor-Associated Inflammation
4. Promotion of Tissue Regeneration

How
Chlorine Dioxide Destroys Tumors

Advantages of the Intratumoral Chlorine Dioxide Ablation System
The system is designed for localized intratumoral ablation of solid tumors, enabling direct targeting of tumor tissue through controlled delivery of chlorine dioxide. Its localized, procedure-based approach differentiates it from systemic treatment modalities.
No Side Effects
Chlorine dioxide, as a strong oxidizer, reacts immediately with tumor tissue, breaking down into chloride ions and water without producing harmful byproducts. The precise injection avoids damage to surrounding healthy tissues.
Rapid and Extensive Tumor Necrosis Without Liquefaction
Chlorine dioxide destroys tumor cells instantly upon contact, with tumor necrosis observable within an hour via ultrasound or CT. It also disrupts tumor blood vessels, causing secondary necrosis. The tumor retains its structure without undergoing liquefactive necrosis, preserving its integrity.
Effective Regardless of Tumor Size or Number
With modern medical techniques, each tumor injection only takes a few minutes. The therapy can target tumors of any size or number, and multiple injections can be administered to large or metastatic tumors, simply requiring a longer treatment duration.
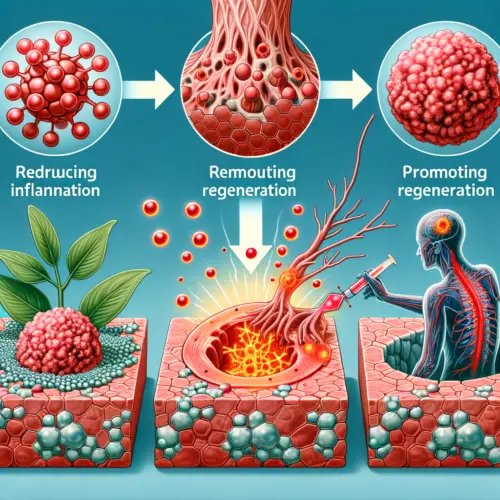
Reduces Inflammation, Prevents Infection, and Promotes Regeneration
In addition to rapidly destroying tumors, chlorine dioxide eliminates inflammation, preventing infection, and accelerates tissue healing. This makes it suitable for cancer patients at any stage, facilitating quick recovery and effectively curing cancer by removing all its detrimental effects.
2010 - Initial Contact with Chlorine Dioxide (ClO₂)
Relation to Intra-Tumoral ClO₂ Therapy: User first explored chlorine dioxide through self-experimentation and small-scale clinical research. This early work laid the foundation for later patent applications and the development of the intra-tumoral ClO₂ therapy.
Timeline of Intra-Tumoral Chlorine Dioxide Therapy Development
2011 - First Patent Filed for Chlorine Dioxide Applications
Relation to Intra-Tumoral ClO₂ Therapy: Patent related to chlorine dioxide’s potential in treating tumors and other conditions. This step was the first in developing a therapeutic protocol, culminating in the intra-tumoral ClO₂ therapy for cancer.
2012 - Patent Application for Stem Cell Activation and Other Applications
Relation to Intra-Tumoral ClO₂ Therapy: The patent expanded chlorine dioxide’s therapeutic potential to stem cell activation and anti-aging, indirectly supporting the therapeutic mechanisms that would later be applied to cancer treatment.
2014 - Second Patent Filed for Cell Apoptosis Induction
Relation to Intra-Tumoral ClO₂ Therapy: This patent further explored the ability of chlorine dioxide to induce cell apoptosis, a key mechanism in cancer treatment through intra-tumoral ClO₂ injections.
2016 - Completion of Preclinical Research
Relation to Intra-Tumoral ClO₂ Therapy: The successful preclinical studies confirmed the safety and efficacy of chlorine dioxide injections for tumor targeting, providing strong evidence for moving forward with human trials.
2023 - Writing “The Chlorine Dioxide Miracle”
Relation to Intra-Tumoral ClO₂ Therapy: The book outlines chlorine dioxide’s therapeutic potential, including its ability to target tumors, a foundational principle in the intra-tumoral ClO₂ therapy for cancer treatment.
May 2024 - First Human Breast Cancer Trial
Relation to Intra-Tumoral ClO₂ Therapy: This marks the first clinical application of intra-tumoral ClO₂ therapy in a breast cancer patient, yielding ideal results and paving the way for broader clinical trials.
December 2024 - Technical Licensing Agreements with Clinics in Germany, Mexico, Brazil, and the Philippines
Relation to Intra-Tumoral ClO₂ Therapy: These agreements allowed the therapy to be offered to patients, starting with a German clinic where 5 patients were treated with remarkable results in 2024.
2025 - Beginning of Paid Treatment and Continued Success
Relation to Intra-Tumoral ClO₂ Therapy: After continued success in 2024, the therapy began to be offered as a paid treatment in 2025, solidifying its status as a revolutionary cancer treatment.
The Chlorine Dioxide Tumor Destruction Process
Chlorine dioxide injection is a quick, safe, and adjustable treatment. It reacts immediately to produce only chlorine ions and water, with no harmful byproducts or significant side effects. The injection takes just a few minutes, and the dosage can be tailored to treat various cancers, offering a flexible, effective solution with minimal risk.
Direct Killing of Tumor Cells
When chlorine dioxide is injected into the tumor, it undergoes an oxidation reaction, producing chlorine ions and water. This reaction happens rapidly, leading to the breakdown and death of cancer cells, which helps reduce the tumor size.
Benefit: The therapy works quickly, shrinking the tumor by effectively killing cancer cells, and it has no side effects.
Penetration and Destruction of Tumor Blood Vessels
Chlorine dioxide reaches and damages the tumor’s blood vessels. By breaking down the blood vessels, it cuts off the tumor’s supply of oxygen and nutrients, essentially starving the tumor cells.
Benefit: This process further weakens the tumor by depriving it of vital resources, making it harder for the remaining cancer cells to survive.
Eliminating Inflammation and Promoting Regeneration
Chlorine dioxide reduces inflammation around the tumor, which helps the body heal. This promotes tissue regeneration, allowing the body to recover faster from the damage caused by the tumor.
Benefit: Reducing inflammation speeds up recovery and helps restore the body’s normal function.